Discovery and Characterization of Novel Nonsubstrate and Substrate NAMPT Inhibitors.
Wilsbacher, J.L., Cheng, M., Cheng, D., Trammell, S.A.J., Shi, Y., Guo, J., Koeniger, S.L., Kovar, P.J., He, Y., Selvaraju, S., Heyman, H.R., Sorensen, B.K., Clark, R.F., Hansen, T.M., Longenecker, K.L., Raich, D., Korepanova, A.V., Cepa, S., Towne, D.L., Abraham, V.C., Tang, H., Richardson, P.L., McLoughlin, S.M., Badagnani, I., Curtin, M.L., Michaelides, M.R., Maag, D., Buchanan, F.G., Chiang, G.G., Gao, W., Rosenberg, S.H., Brenner, C., Tse, C.(2017) Mol Cancer Ther 16: 1236-1245
- PubMed: 28468779
- DOI: https://doi.org/10.1158/1535-7163.MCT-16-0819
- Primary Citation of Related Structures:
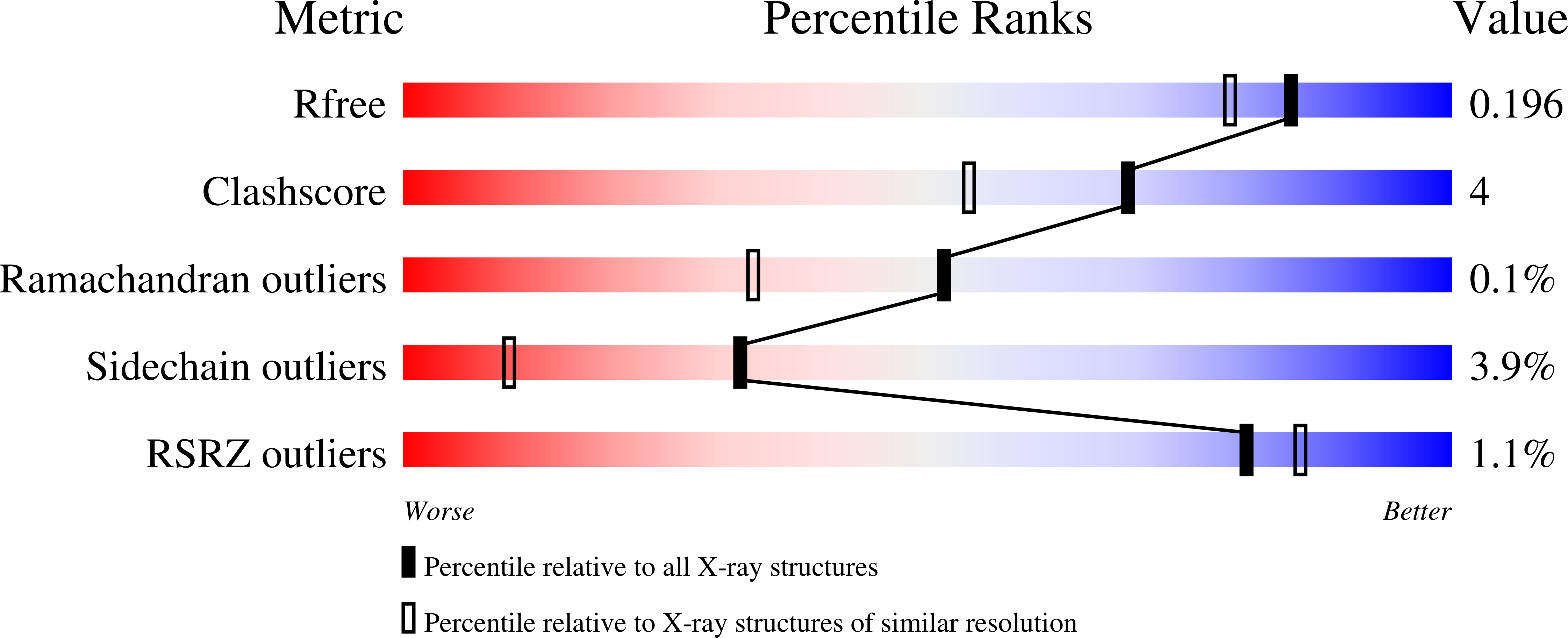
5U2M, 5U2N - PubMed Abstract:
Cancer cells are highly reliant on NAD + -dependent processes, including glucose metabolism, calcium signaling, DNA repair, and regulation of gene expression. Nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD + salvage from nicotinamide, has been investigated as a target for anticancer therapy. Known NAMPT inhibitors with potent cell activity are composed of a nitrogen-containing aromatic group, which is phosphoribosylated by the enzyme. Here, we identified two novel types of NAM-competitive NAMPT inhibitors, only one of which contains a modifiable, aromatic nitrogen that could be a phosphoribosyl acceptor. Both types of compound effectively deplete cellular NAD + , and subsequently ATP, and produce cell death when NAMPT is inhibited in cultured cells for more than 48 hours. Careful characterization of the kinetics of NAMPT inhibition in vivo allowed us to optimize dosing to produce sufficient NAD + depletion over time that resulted in efficacy in an HCT116 xenograft model. Our data demonstrate that direct phosphoribosylation of competitive inhibitors by the NAMPT enzyme is not required for potent in vitro cellular activity or in vivo antitumor efficacy. Mol Cancer Ther; 16(7); 1236-45. ©2017 AACR .
Organizational Affiliation:
AbbVie Inc., North Chicago, Illinois. julie.wilsbacher@abbvie.com.